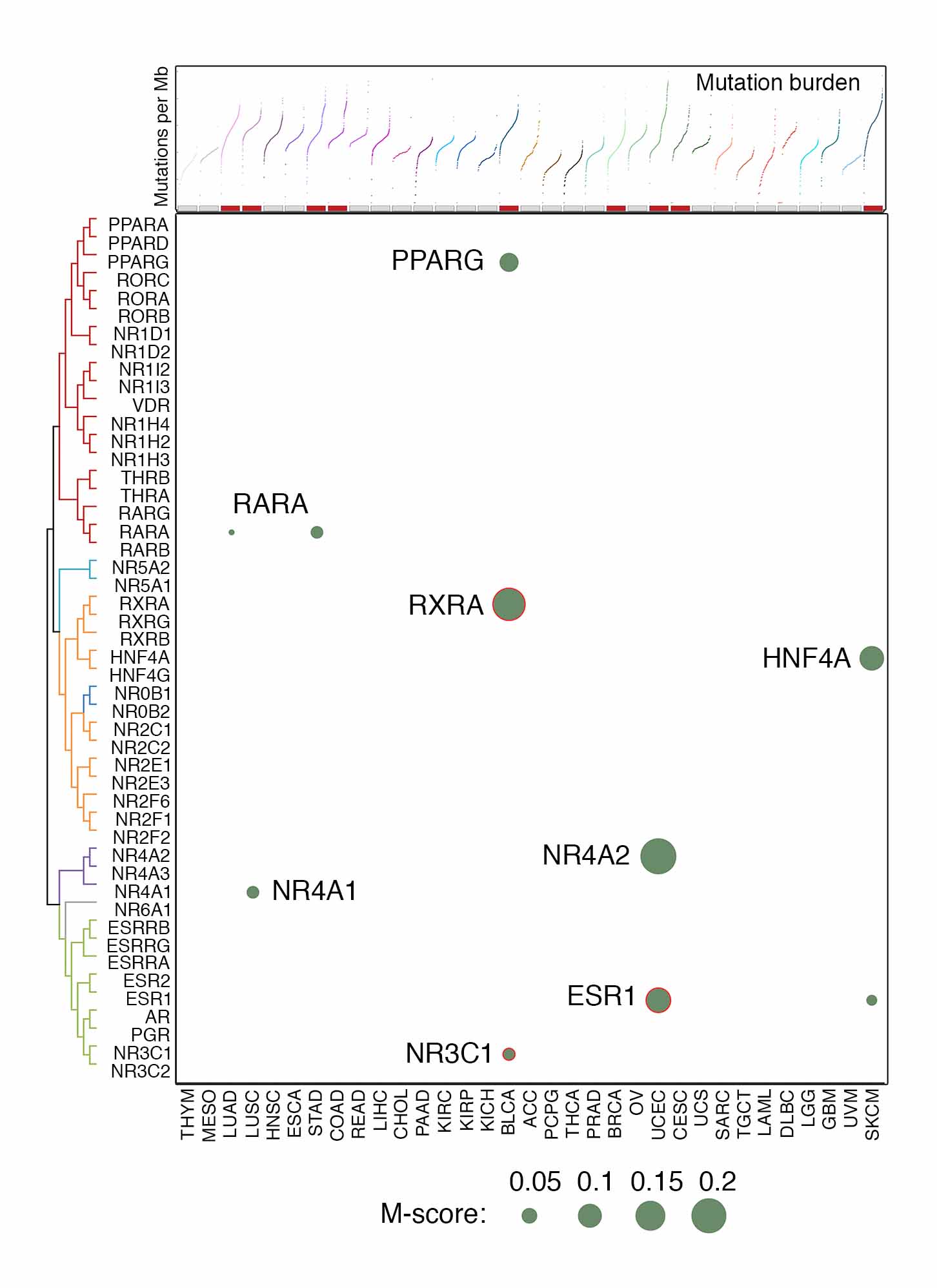
To characterize somatic mutations of NR genes in cancers, we analyzed whole exome sequencing (WES) profiles from TCGA (Dataset 3). The mutation call set generated by an ensemble calling strategy was retrieved from the Multi-Center Mutation Calling in Multiple Cancers (MC3 project) and used in our analysis. We used an integrated computational approach to estimate the recurrent mutations that have significantly higher mutation rates than the background mutations in the cancer genomes and represent the presence of selective pressure for these mutations during tumor evolution. A mutation score (M-score), which considered both the mutation index and the frequency of mutations across samples, was estimated for each NR gene in a given cancer type (Dataset 10). A total of 8 NRs with recurrent mutations were initially identified: 6 of them were found in a single cancer type, and 2 were observed in multiple cancer types (Figure 4A). Although recurrent mutations of ESR1 and AR have been reported in advanced metastatic breast and prostate cancers, low mutation frequencies of ESR1 (0.84%, 9 cases) and AR (0.4%, 2 cases) mutations were observed in BRCA and PRAD of the TCGA cohorts, which were mainly treatment-naïve primary tumors. After excluding the hypermutated samples of each cancer type to avoid noise from high background mutation rates, only 3 NRs passed the threshold in bladder urothelial carcinoma (BLCA [RXRA, NR3C1]) and UCEC (ESR1). Given the high frequency of truncating and missense mutations (10.6%) for NR4A2 in UCEC and strong functional evidence for PPARG mutations in BLCA, these 2 NRs were also included in our downstream analyses together with RXRA, NR3C1, and ESR1. The most common mutation type in these 5 NRs were missense mutations, accounting for 61.9% to 82.1% of all mutations, respectively. NR4A2 had the highest fraction (15.3%) of truncating mutations, while ESR1 showed the highest portions of missense (82.1%) and in-frame (5.1%) mutations (Figure 4B). Additionally, analysis by ABSOLUTE algorithm indicated that more than half of the mutations in these NRs were clonal and early-stage mutations, and a low percentage of homozygous mutations were observed (Figure 4C). The higher fractions of clonal mutations in NRs, especially in NR3C1 in BLCA, suggests that these NRs may contribute to tumorigenesis during tumor evolution, serving as actionable genomic events. We also characterized the distribution of mutations along the NR gene bodies. NR mutations were widely and spatially distributed across the entire coding sequences. Due to the limited number of samples harboring these mutations in the TCGA cohort, no mutations were found to be significantly concentrated within a specific region, although RXRA showed a higher frequency of mutation S427F/Y. Previous studies by independent groups suggested 5 potential functional mutations in RXRA, PPARG and ESR1 (Figure 4D and 4E). For example, S427F/Y located in the LBD of the RXRA gene was reported as a hotspot mutation that affects the RXRA/PPARG heterodimer formation, thereby activating ligand-independent PPARG signaling. Additionally, although the H494Y mutation of PPARG is not in the LBD region, it has been identified as a 3D-clustered hotspot and predicted to be oncogenic. Three functional mutations were also reported in ESR1, including mutations on Y537 and D538 that have been demonstrated to serve as oncogenic mutations in BRCA. Notably, significant mutual exclusivity between PPARG copy number gain and somatic mutation was observed in BLCA (p<0.001), indicating their mutual roles in activating downstream signaling (Figure 4F). Additionally, mutual exclusivity of NR mutations was also identified. For example, mutations of RXRA, PPARG, and NR3C1 were significantly mutually exclusive in BLCA, and mutual exclusivity of ESR1 and NR4A2 mutations were found in UCEC. Taken together, although exhibiting relatively low frequencies, recurrent mutations were identified in NRs, including RXRA, NR3C1, ESR1, PPARG and NR4A2. Notably, most TCGA tumors were collected from treatment-naïve primary tumors. Mutations in NRs may further increase during tumor progression (e.g., ESR1 in breast cancer, and AR in prostate cancer).