Cancer remains a leading cause of mortality worldwide, necessitating the continuous exploration of novel biomarkers and therapeutic targets. Nuclear receptors (NRs), a family of 48 ligand-dependent transcription factors, have emerged as pivotal players in cancer biology.
Their roles in regulating gene expression, cell proliferation, differentiation, and metabolism position them as both diagnostic biomarkers and therapeutic targets in oncology.
This article delves into the significance of nuclear receptors in cancer, highlighting their potential in diagnosis, prognosis, and treatment strategies.
Understanding Nuclear Receptors
What Are Nuclear Receptors?
Nuclear receptors are intracellular proteins that, upon binding to specific ligands such as hormones, vitamins, or other small molecules, modulate the expression of target genes.
They play crucial roles in various physiological processes, including development, metabolism, and immune response.
In the context of cancer, dysregulation of nuclear receptor signaling can lead to uncontrolled cell growth and tumor progression.
Classification of Nuclear Receptors
Nuclear receptors are classified into several subfamilies based on their structural and functional characteristics:
- Steroid Hormone Receptors: Include estrogen receptor (ER), androgen receptor (AR), progesterone receptor (PR), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR).
- Thyroid Hormone Receptors: Thyroid hormone receptor (TR).
- Retinoic Acid Receptors: Retinoic acid receptor (RAR) and retinoid X receptor (RXR.
- Orphan Receptors: Receptors with unknown endogenous ligands, such as peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs).
- Nuclear Hormone Receptors: Including vitamin D receptor (VDR) and farnesoid X receptor (FXR).
Nuclear Receptors as Cancer Biomarkers
Diagnostic Biomarkers
Nuclear receptors serve as valuable diagnostic tools in oncology. For instance, the expression of estrogen and progesterone receptors in breast cancer tissues aids in subclassifying tumors into distinct molecular subtypes, influencing treatment decisions.
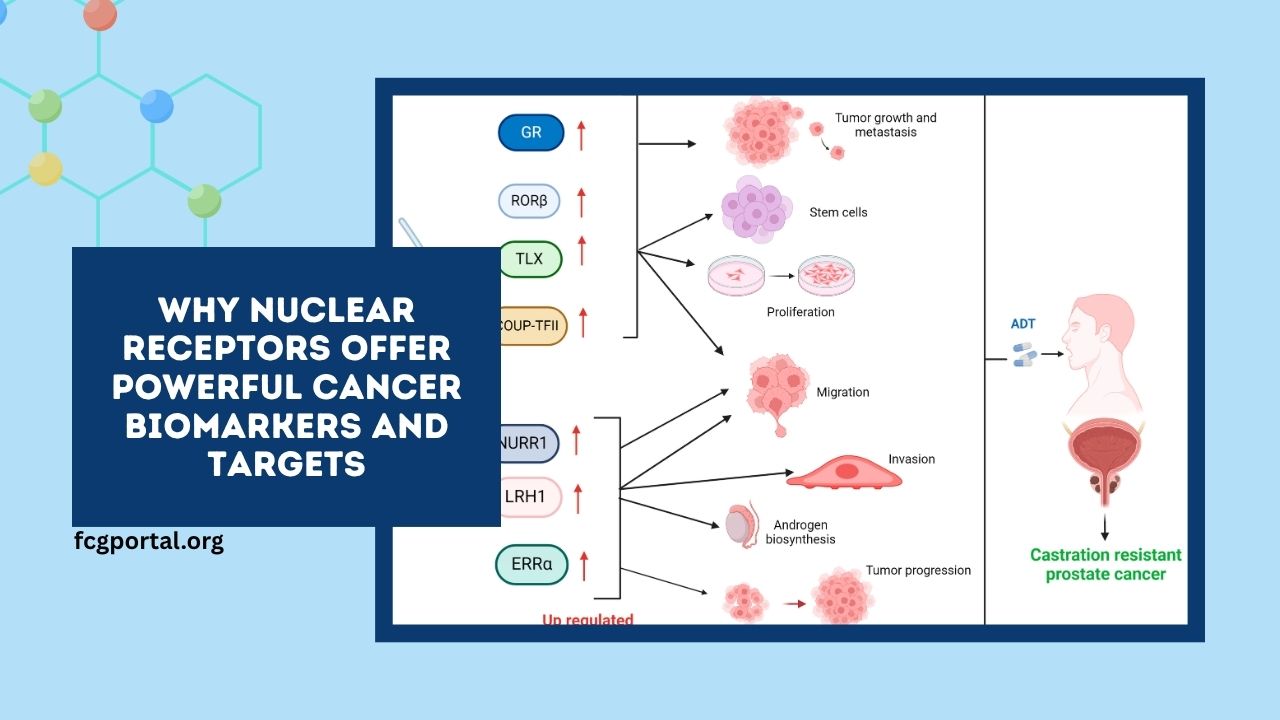
Similarly, androgen receptor expression in prostate cancer is utilized to determine the appropriate therapeutic approach.
Prognostic Biomarkers
The expression levels of certain nuclear receptors correlate with patient prognosis.
High levels of ER and PR are associated with a favorable prognosis in breast cancer, while low expression of VDR in colorectal cancer has been linked to poor outcomes.
These correlations assist clinicians in predicting disease progression and tailoring personalized treatment plans.
Nuclear Receptors as Therapeutic Targets
Targeted Therapies
Nuclear receptors are integral to the development of targeted therapies in cancer treatment.
Selective estrogen receptor modulators (SERMs) like tamoxifen are employed in ER-positive breast cancer, while AR antagonists such as enzalutamide are used in castration-resistant prostate cancer.
These therapies aim to block the activity of specific nuclear receptors, thereby inhibiting tumor growth.
Emerging Therapeutic Agents
Research is ongoing to develop novel agents targeting nuclear receptors.
For example, PPAR agonists are being investigated for their potential to induce differentiation in leukemia cells, while VDR ligands are explored for their role in modulating immune responses in various cancers.
These emerging agents hold promise for expanding the therapeutic arsenal against cancer.
Nuclear Receptors in Tumor Microenvironment
Beyond tumor cells, nuclear receptors influence the tumor microenvironment, affecting cancer progression and therapy response. For instance, PPARs modulate inflammation and immune cell infiltration, while GR regulates stress responses and cell survival pathways.
Understanding the role of nuclear receptors in the tumor microenvironment is crucial for developing strategies to overcome resistance and enhance treatment efficacy.
Challenges and Future Directions
Drug Resistance
Despite the therapeutic potential of targeting nuclear receptors, challenges such as acquired drug resistance pose significant hurdles.
Mutations in nuclear receptor genes or alterations in co-regulatory proteins can lead to resistance, necessitating the development of next-generation agents that can overcome these obstacles.
Personalized Medicine
The heterogeneity of nuclear receptor expression across different cancer types and individual patients underscores the need for personalized treatment approaches.
Tailoring therapies based on specific nuclear receptor profiles can improve treatment outcomes and minimize adverse effects.
Nuclear receptors play a pivotal role in cancer biology, serving as both biomarkers for diagnosis and prognosis and as targets for therapeutic intervention.
Their involvement in various aspects of cancer progression, including cell proliferation, differentiation, and metastasis, makes them attractive candidates for targeted therapies.
Continued research into the functions of nuclear receptors and the development of agents that modulate their activity will be instrumental in advancing cancer treatment and improving patient outcomes.
FAQs
What are nuclear receptors, and how do they relate to cancer?
Nuclear receptors are a family of 48 transcription factors that regulate gene expression in response to specific ligands. In cancer, dysregulation of nuclear receptors can lead to uncontrolled cell growth and tumor progression, making them critical targets for diagnosis and therapy.
How are nuclear receptors used as cancer biomarkers?
Nuclear receptors serve as diagnostic biomarkers by indicating specific cancer subtypes, such as ER-positive or AR-positive cancers. They also function as prognostic biomarkers, with their expression levels correlating with patient outcomes and guiding treatment decisions.
What challenges exist in targeting nuclear receptors for cancer therapy?
Challenges include the development of drug resistance due to mutations in nuclear receptor genes or associated co-regulatory proteins. Additionally, the heterogeneity of nuclear receptor expression across different cancers necessitates personalized treatment approaches to enhance therapeutic efficacy.