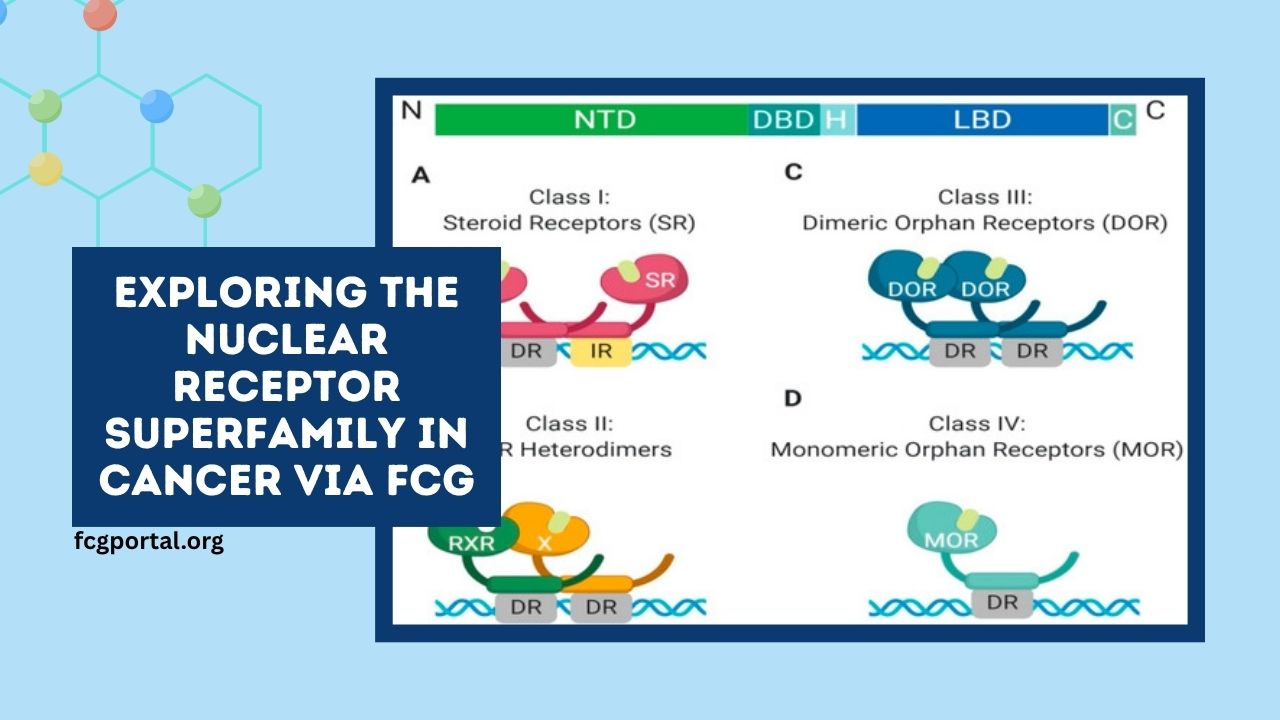
The nuclear receptor superfamily comprises 48 ligand-activated transcription factors that regulate a myriad of physiological processes, including metabolism, development, and immune responses.
In the context of cancer, these receptors influence tumor initiation, progression, and response to therapies.
Recent studies have highlighted the interplay between nuclear receptors and Fc gamma receptors (FcγRs), which are pivotal in mediating immune responses.
This article delves into the intricate relationship between these two families of receptors and their collective impact on cancer biology.
Understanding the Nuclear Receptor Superfamily
Structure and Classification
Nuclear receptors are characterized by:
- DNA-binding domain (DBD): Facilitates binding to specific DNA sequences known as hormone response elements.
- Ligand-binding domain (LBD): Binds to specific ligands, leading to conformational changes that modulate gene expression.
- Transactivation domain: Interacts with coactivators or corepressors to enhance or suppress transcription.
These receptors are classified into seven groups based on structural and functional similarities:
- Class I: Steroid hormone receptors (e.g., estrogen receptor, androgen receptor)
- Class II: Thyroid hormone, retinoic acid, and vitamin D receptors
- Class III: Orphan receptors with unidentified ligands
- Class IV: Nerve growth factor IB-like receptors
- Class V: Steroidogenic factor-like receptors
- Class VI: Germ cell nuclear factor-like receptors
- Class 0: Orphan receptors without a known ligand-binding domain
Role in Cancer
Nuclear receptors influence cancer through:
- Gene expression modulation: Regulating genes involved in cell proliferation, apoptosis, and differentiation.
- Metabolic reprogramming: Altering metabolic pathways to support tumor growth.
- Immune modulation: Influencing the tumor microenvironment and immune cell infiltration.
Fcγ Receptors: Mediators of Immune Response
Structure and Function
Fcγ receptors are cell surface proteins that bind to the Fc region of immunoglobulin G (IgG) antibodies. They are classified into:
- Activatory receptors: FcγRI, FcγRIIA, FcγRIIIA
- Inhibitory receptors: FcγRIIB
These receptors mediate various immune responses, including:
- Phagocytosis: Engulfment of pathogens or tumor cells.
- Antibody-dependent cellular cytotoxicity (ADCC): Destruction of antibody-coated target cells.
- Cytokine release: Secretion of pro-inflammatory cytokines.
Fcγ Receptors in Cancer
In cancer, FcγRs play dual roles:
- Tumor promotion: By facilitating immune evasion and promoting tumor cell survival.
- Tumor suppression: Through ADCC and phagocytosis of tumor cells.
The balance between these opposing effects is influenced by the expression levels and activation states of FcγRs on immune cells.
Interplay Between Nuclear Receptors and Fcγ Receptors in Cancer
Regulation of FcγR Expression by Nuclear Receptors
Nuclear receptors can modulate the expression of FcγRs:
- Glucocorticoid receptor (GR): Activation can suppress FcγRI expression on macrophages, reducing phagocytosis.
- Retinoic acid receptor (RAR): Influences the expression of FcγRs on dendritic cells, affecting antigen presentation and immune activation.
Impact on Tumor Immunity
The interaction between nuclear receptors and FcγRs affects:
- Immune cell infiltration: Altering the recruitment of immune cells to the tumor site.
- Cytokine production: Modifying the inflammatory milieu within the tumor microenvironment.
- Therapeutic response: Influencing the efficacy of antibody-based therapies.
Clinical Implications and Therapeutic Strategies
Targeting Nuclear Receptors in Cancer Therapy
Modulating nuclear receptor activity offers therapeutic potential:
- Selective nuclear receptor modulators (SNRMs): Drugs that specifically activate or inhibit nuclear receptors.
- Combination therapies: Using SNRMs alongside traditional therapies to enhance efficacy.
Enhancing FcγR-Mediated Immune Responses
Strategies to boost FcγR activity include:
- Fc engineering: Designing antibodies with enhanced affinity for FcγRs.
- Immune checkpoint inhibitors: Targeting pathways that suppress FcγR-mediated responses.
Challenges and Future Directions
- Resistance mechanisms: Tumor cells may adapt to evade FcγR-mediated immune responses.
- Heterogeneity: Variability in receptor expression among patients complicates treatment strategies.
Summary of Nuclear Receptors and Their Role in Cancer
| Nuclear Receptor | Function in Cancer | Impact on FcγR |
|---|---|---|
| Estrogen Receptor (ER) | Promotes tumor cell proliferation | Modulates immune cell recruitment |
| Androgen Receptor (AR) | Supports survival of prostate cancer cells | Alters FcγR expression on macrophages |
| Retinoic Acid Receptor (RAR) | Induces differentiation of tumor cells | Affects dendritic cell function |
| Peroxisome Proliferator-Activated Receptor (PPAR) | Regulates lipid metabolism in tumors | Influences macrophage polarization |
| Glucocorticoid Receptor (GR) | Mediates stress response in tumors | Suppresses FcγRI expression on macrophages |
The interplay between the nuclear receptor superfamily and Fcγ receptors is a critical aspect of cancer biology.
Understanding this interaction opens avenues for novel therapeutic strategies aimed at modulating immune responses and improving patient outcomes.
Future research focusing on this axis may lead to more effective and personalized cancer treatments.
FAQs
How do nuclear receptors influence cancer progression?
Nuclear receptors regulate genes involved in cell growth, apoptosis, and metabolism, thereby influencing tumor initiation and progression.
What is the role of Fcγ receptors in cancer immunity?
Fcγ receptors mediate immune responses such as phagocytosis and ADCC, which can either promote or suppress tumor growth depending on their activation.
Can targeting nuclear receptors enhance cancer therapy?
Yes, modulating nuclear receptor activity can improve therapeutic outcomes by altering tumor biology and enhancing immune responses.